menu
menu
Development of ²¹²Pb-based Radio-DARPin in therapy (RDT) for the treatment of mesothelin (MSLN)-positive solid tumors
Abstract:
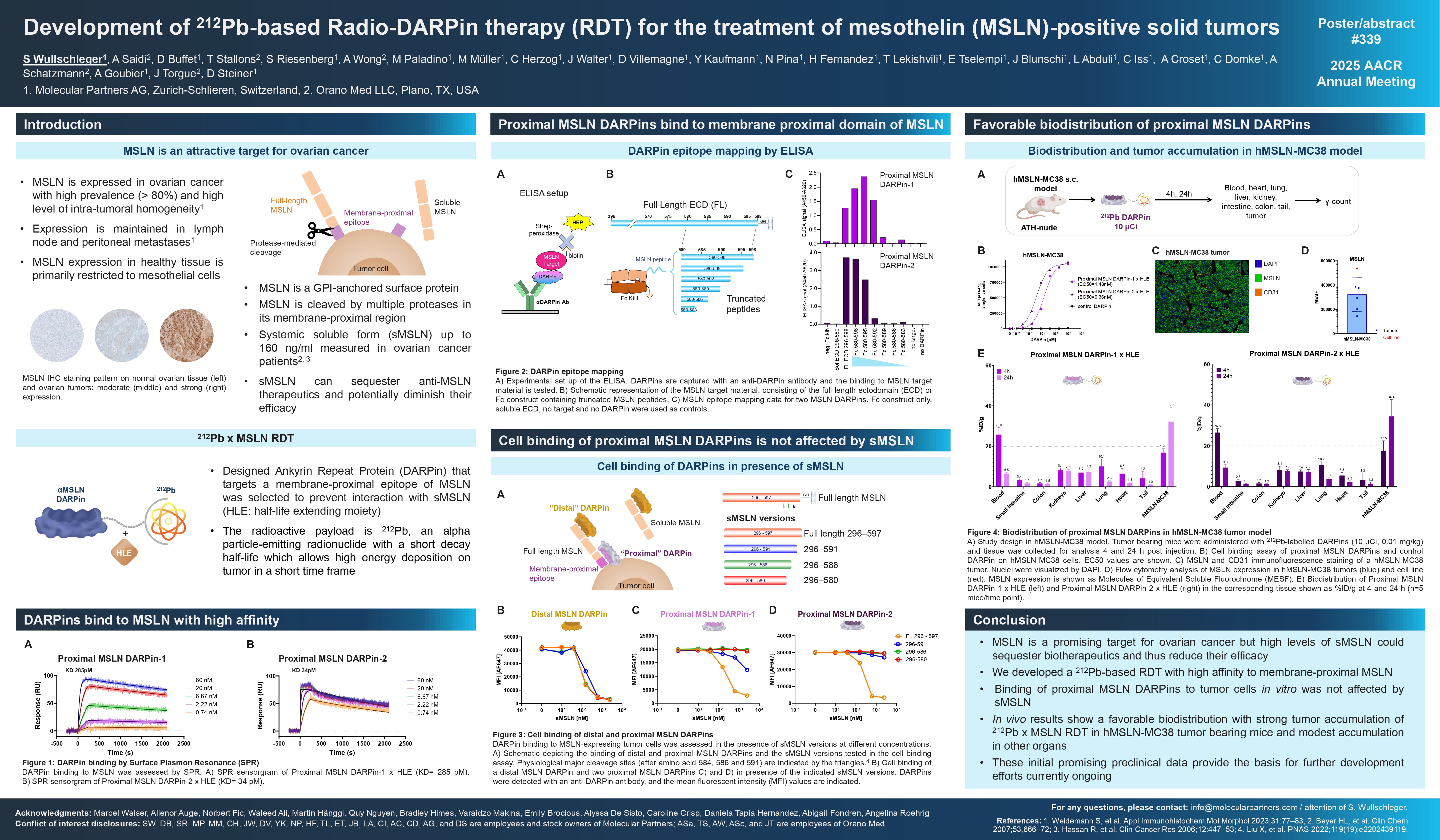
Radioligand therapies have emerged as promising treatment modality for certain cancers. This therapeutic approach relies on a suitable tumor-targeting moiety labelled with a radioactive payload to allow the delivery of the radionuclide to cancer cells with high specificity. Due to its expression profile in tumor vs healthy tissues, MSLN is an attractive protein for targeted therapies. MSLN is a GPI-anchored membrane protein which is overexpressed in many tumor types, including ovarian cancer and pancreatic adenocarcinoma. Therapeutic approaches targeting MSLN have encountered obstacles due to the high levels of its proteolytically cleaved soluble form (sMSLN), which can sequester anti-MSLN therapeutics and potentially diminish their efficacy. To circumvent this caveat, we sought to generate a Designed Ankyrin Repeat Protein (DARPin) that binds to a membrane proximal epitope of MSLN and therefore avoids binding to sMSLN. Thanks to their small size and high affinity, DARPins are ideal vectors for targeted radiotherapies, dubbed as Radio-DARPin therapies (RDT). As radioactive payload we used ²¹²Pb, an alpha particle-emitting radionuclide with a short half-life and a favorable decay profile which allows high energy deposition on tumor in a short time frame. Herein we report for the first time the development and initial preclinical characterization of our anti-MSLN ²¹²Pb-RDT approach. In vitro assays revealed that the membrane proximal MSLN targeting DARPin specifically interacts with MSLN expressed on cancer cells with high affinity, and cell binding was not affected by the presence of sMSLN. The DARPin was then labelled with ²¹²Pb and its preclinical pharmacokinetic and biodistribution profile was evaluated in MSLN-expressing xenograft tumor models. In these tumor bearing mice, the anti-MSLN ²¹²Pb Radio-DARPin demonstrated substantial uptake into MSLN-positive tumors, while other organs only showed limited accumulation. We designed an approach to specifically target MSLN expressed on cancer cells employing DARPin technology combined with ²¹²Pb. Our early preclinical results indicative of a favorable biodistribution profile of the ²¹²Pb labelled MSLN DARPin motivate further development of this RDT for the treatment of patients with MSLN-positive solid tumors.
Conclusion:
- MSLN is a promising target for ovarian cancer but high levels of sMSLN could sequester biotherapeutics and thus reduce their efficacy
- We developed a 212Pb-based RDT with high affinity to membrane-proximal MSLN
- Binding of proximal MSLN DARPins to tumor cells in vitro was not affected by sMSLN
- In vivo results show a favorable biodistribution with strong tumor accumulation of 212Pb x MSLN RDT in hMSLN-MC38 tumor bearing mice and modest accumulation in other organs
- These initial promising preclinical data provide the basis for further development efforts currently ongoing