 menu
menu
Lead-212 Alpha Therapy
Alpha emitters are considered as the most powerful payloads to be found for targeted therapies
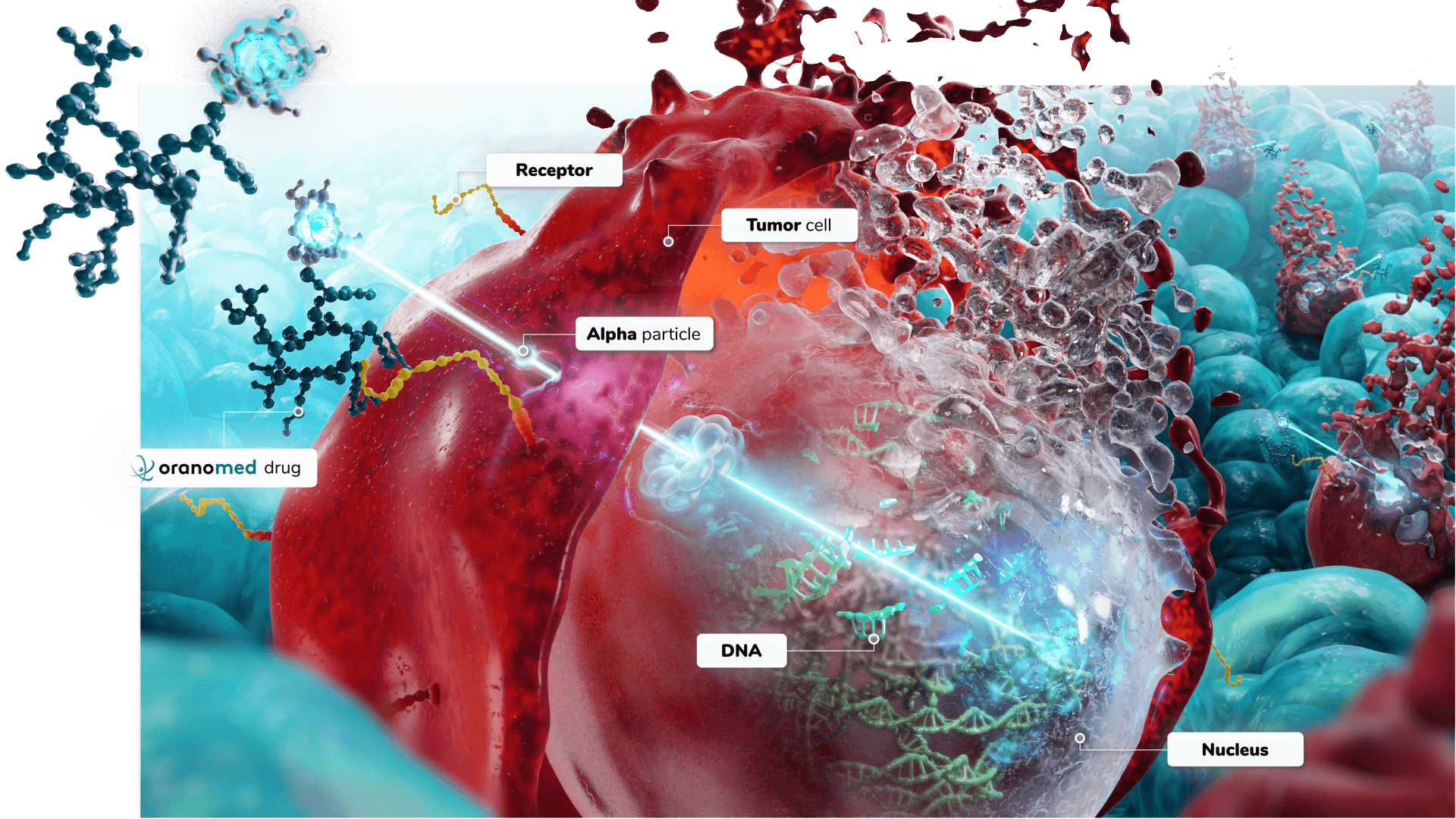
A powerful way to treat cancer

- an atom of 212Pb, an in vivo generator of alpha emitters,
- a biological vector (peptide, antibody, etc.) specifically targeting tumor cells,
- a chelating agent to bind the 212Pb to the vector.
212Pb can be combined with a wide range of targeting vectors, thereby vastly increasing the potential range of applications in oncology.
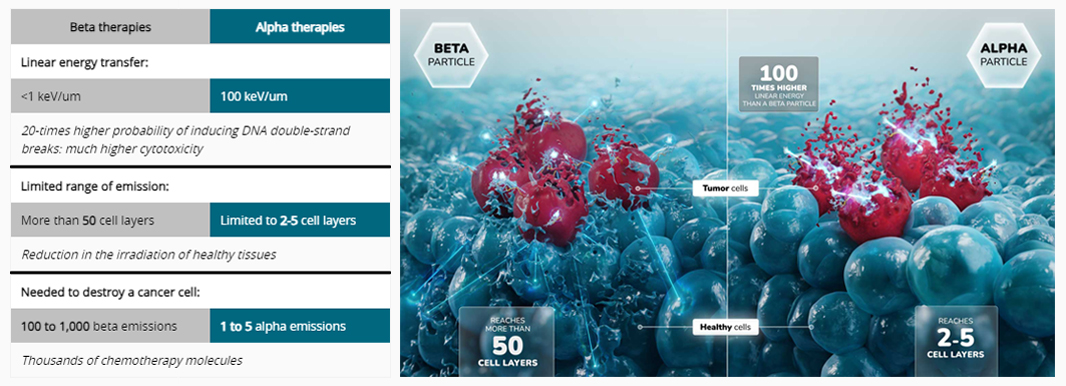
Superiority of targeted alpha therapy in the fight against cancer
Two types of isotopes can be used in radioligand therapies: emitters of alpha or beta radiation. Currently, only beta therapies are commercially available. However, alpha particles have two key benefits for applications in oncology:
1 - Improved biological efficacy
Alpha decay consists of the emission of a helium nucleus (alpha particle) together with linear energy transfer which is 100 times higher than that of beta radiation. The alpha radiation thus causes irreparable double-strand breaks in the DNA of cells in immediate proximity to the emission while beta radiation has more of a tendency to cause single-strand breaks. This specific mechanism of action of alpha emissions hinders the development of processes of resistance or immune escape in tumor cells.
As a result, alpha emitters are considered as the most powerful payloads to be found for targeted therapies with fewer than five particles needed to kill a cancer cell versus hundreds of beta emitting isotopes or thousands of chemotherapy toxins. Another advantage of TAT is that it does not require internalization of vectors to be effective.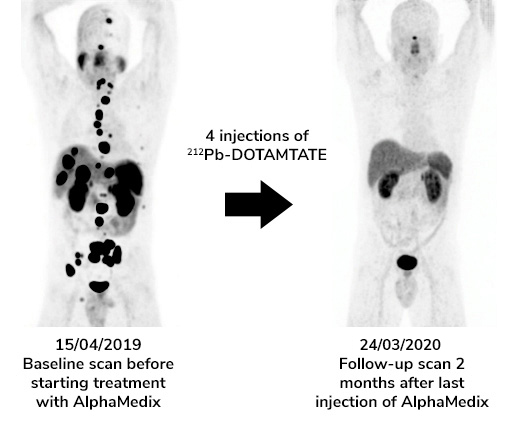
2 - Toxicity limited thanks to short range of emission
The alpha particles only travel over a very limited distance into the tissues: only 2 to 5 cell layers (compared to more than 50 with beta radiation). They thus deposit a very large amount of energy over a very short distance. This results in an increased cytotoxic potential toward cancer cells while limiting toxicity to nearby healthy cells. This very short range of emission also makes alpha emitters particularly suitable for the treatment of micro-metastases which are difficult to target with other molecules.
Mechanism of action of lead-212 alpha therapy
Why lead-212 ?
Lead-212 has all the qualities required for application in radioligand therapies:
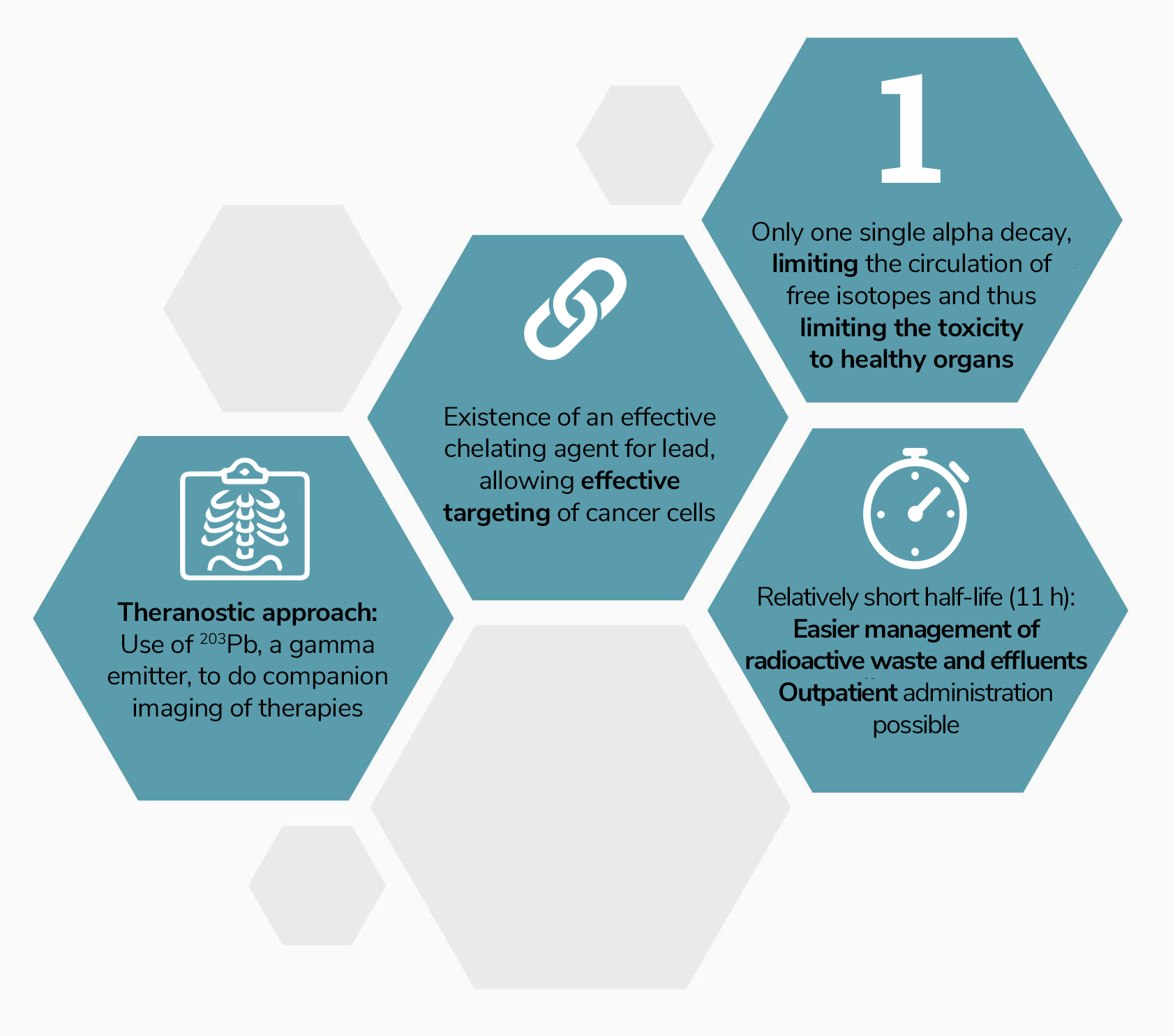
- its half-life of nearly 11 hours facilitates the management of radioactive waste and effluents and allows treatments to be administered on an outpatient basis, placing less constraints on hospitals;
- it only has a single alpha emission in its decay chain, limiting the circulation of free radioactive isotopes (not chelated to the vector) and thus the toxicity to healthy organs;
- it has a particularly stable chelating agent, allowing effective targeting of cancer cells;
- Another isotope of lead, 203Pb, is an imageable gamma emitter, allowing the development of theranostic approaches with 203Pb / 212Pb.
Moreover, Orano Med has developed an entirely chemical production process. This makes it more reliable and less costly
than other processes usually used for the production of radioisotopes by cyclotron or in a nuclear reactor.
Preclinical and clinical development


- A preclinical laboratory dedicated to the development of 212Pb Targeted Alpha Therapy treatments in the USA (Plano, Texas). It is fully equipped to conduct the necessary preclinical studies to develop new molecules as well as to perform activities of peptide synthesis, bioconjugation, radiolabeling and analytical testing. Its subsidiary Macrocylics shares the same premises and manufactures on site the chelatants.
- A second preclinical laboratory in France in partnership with Roche (Razès, Haute-Vienne), to develop novel, advanced alpha radioimmunotherapy techniques.
Two of Orano Med's compounds are currently in the clinic:
- A phase 2 clinical trial on neuroendocrine tumors with AlphaMedixTM is completed.
- A phase 1 clinical trial on different types of solid tumors with an anti-GRPR peptide as a targeting molecule.