 menu
menu
ESMO: AlphaMedix™ phase 2 data support first-in-class potential of new targeted alpha therapy in gastroenteropancreatic neuroendocrine tumors
- New AlphaMedixTM data showed sustained and clinically meaningful responses across both RLT-naïve and RLT-exposed patients with unresectable or metastatic GEP-NETs
- First phase 2 study to evaluate TAT with lead-212 supporting its potential to address high unmet medical needs in difficult-to-treat, rare cancers
- Phase 2 study met all primary efficacy endpoints and was presented across two oral presentations at the 2025 ESMO Congress in Berlin, Germany
New data from the ALPHAMEDIX-02 phase 2 study (clinical study identifier: NCT05153772) evaluating AlphaMedix (212Pb-DOTAMTATE), an investigational somatostatin receptor (SSTR) targeted alpha therapy (TAT) using the lead-212 isotope, underscore the first-in-class potential of this investigational medicine for the treatment of patients with advanced gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Detailed results from the phase 2 study, the first to evaluate a TAT across both radioligand therapy (RLT)-naïve and RLT-exposed patients affected by GEP-NETs, were presented in two oral presentations at the 2025 European Society for Medical Oncology (ESMO) Congress, Berlin, Germany.
“Lead-212-based Targeted Alpha Therapy (TAT) could have a transformative impact across a broad range of solid tumors. With AlphaMedix’s consistent and clinically meaningful responses across both RLT-naïve and RLT-exposed gastroenteropancreatic neuroendocrine tumor (GEP-NET) patients, the positive results underscore its potential in this rare and difficult-to-treat cancer,” said Volker Wagner, MD, PhD, Chief Medical Officer at Orano Med. “These data strongly encourage us to further advance the clinical development of AlphaMedix, and jointly with our partner, Sanofi, make this innovative TAT available to patients in need.”ALPHAMEDIX-02 phase 2 study
ALPHAMEDIX-02 is a phase 2, open-label, multicenter study evaluating the efficacy and safety of AlphaMedix in patients with unresectable or metastatic SSTR+ GEP-NETs. The study included two cohorts evaluating RLT-naïve and RLT-exposed patients. The primary efficacy endpoint across both cohorts was the overall response rate (ORR). Secondary endpoints included progression-free survival (PFS) and overall survival (OS).The study's efficacy endpoints are based on local investigator assessment, per protocol. In addition, a blinded independent central review (BICR) was conducted subsequently. The key efficacy endpoints were met within both the investigator-assessed and BICR results.
The following results were presented:
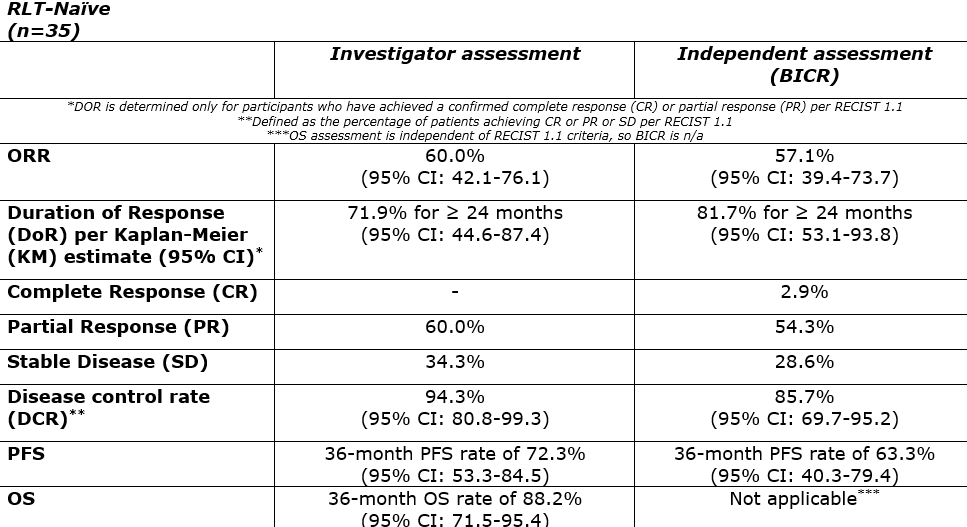
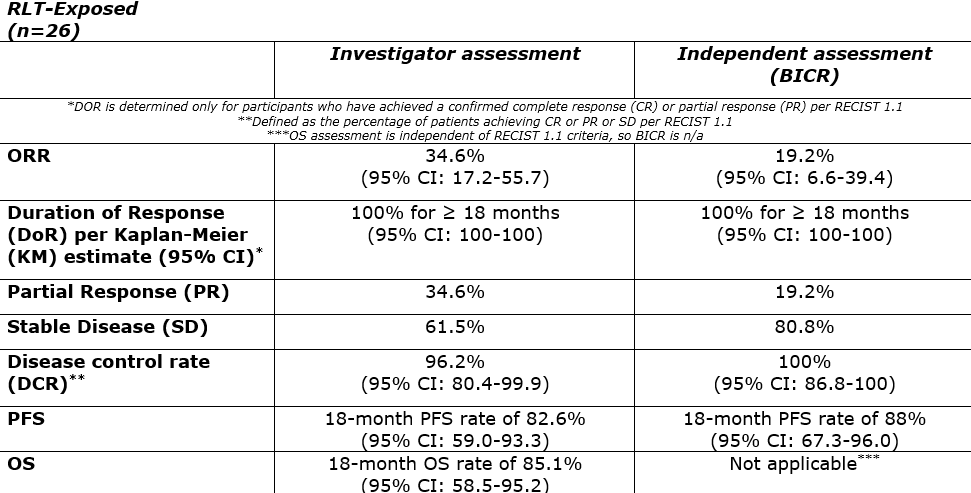
AlphaMedix™ had a similar safety profile across both cohorts. Within the RLT-naïve cohort, 85.7% of patients received all four doses of AlphaMedix, and 84.6% of patients within the RLT-exposed cohort. All GEP-NET patients experienced at least one treatment-emergent adverse event (TEAE). Grade ≥3 TEAEs occurred in 42.3% of RLT-exposed patients and 54.3% of RLT-naïve patients. The most common Grade ≥3 TEAEs in both groups was lymphocyte count decrease (25.7% of RLT-naïve patients and 15.4% of RLT-exposed patients).
“The future of oncology research will be driven by cutting-edge science and next-generation modalities, such as radioligand therapies. AlphaMedix, a promising targeted alpha therapy, embodies the type of solution Sanofi is working to advance,” said Christopher Corsico, MD, Global Head of Development at Sanofi. “We are excited to share these robust scientific findings at ESMO as the data could represent a significant advancement in how we treat gastroenteropancreatic neuroendocrine tumors. As we engage with health authorities and advance the clinical program, we remain focused on bringing this innovative modality to patients who need new treatment options”
Advancing AlphaMedix in GEP-NETs
RLTs, which work by delivering radiation directly to tumor cells, represent an emerging area of oncology research. While current approved beta-emitting RLTs have improved outcomes in patients with GEP-NETs, there remains a critical gap in care, including in those who have progressed following previous RLT.TAT represents an innovative modality in RLT, harnessing high-energy, short-range alpha emissions to precisely target cancer cells while reducing potential exposure to surrounding tissue.
"The results observed in the ALPHAMEDIX-02 trial clearly demonstrate exceptional levels of efficacy for a Targeted Alpha Therapy (TAT), based on current available therapies, in both radioligand therapy (RLT)-naïve and RLT-exposed populations and could potentially set new expectations when treating gastroenteropancreatic neuroendocrine tumor (GEP-NET) patients with RLTs” said Ebrahim Delpassand, MD, Founder and Chairman, CEO of RadioMedix. “For too long, this patient population has experienced inadequate disease control with current approved therapies. This important work provides hope for a new treatment for GEP-NET patients, their caregivers, and their healthcare providers.”
In February 2024, AlphaMedix™ was designated as a breakthrough therapy by the US Food and Drug Administration in RLT-naïve patients with unresectable or metastatic GEP-NETs, recognizing the potential clinical benefits of lead-212–based TATs. The ALPHAMEDIX-02 results will form the basis for further discussions with health authorities.
An international phase 3 study to further evaluate AlphaMedix in GEP-NETs is actively being planned. AlphaMedix is an investigational medicine and has not been approved by any regulatory authority.
In September 2024, Sanofi entered an exclusive licensing agreement with Orano Med and RadioMedix to globally commercialize AlphaMedix.
About the ALPHAMEDIX-02 study
ALPHAMEDIX-02 is a phase 2, open-label, multicenter study evaluating the efficacy and safety of AlphaMedix (212Pb-DOTAMTATE) in patients with histologically confirmed unresectable or metastatic GEP-NETs, positive somatostatin analogue imaging and at least one site of measurable disease. The study included two cohorts evaluating RLT-naïve (n=35) and RLT-exposed (n=26) GEP-NET patients. RLT-exposed patients had progressive disease after receiving up to four doses of 177Lu-DOTATATE and received their last dose at least six months prior to Day 1. In both cohorts, AlphaMedix was administered at 67.6 μCi/kg every eight weeks for up to four cycles (6 mCi maximum per cycle). The primary efficacy endpoint across both cohorts was ORR per RECIST 1.1. Secondary endpoints included PFS and OS.
About NETs
NETs are a heterogeneous group of cancers that originate from neuroendocrine cells. These cancers occur mostly in the gastrointestinal tract and pancreas but can also occur in other tissues including the thymus, lung, and other uncommon sites such as the ovaries, heart, and prostate. Most NETs strongly express somatostatin receptors. Despite the global prevalence of NETs increasing each year, it is considered a rare cancer that is estimated to affect approximately 35/100,000 individuals worldwide. In the United States, around 12,000 patients annually are expected to be diagnosed with neuroendocrine tumors, with an average five-year survival rate of 60% at a metastatic stage.
About Orano Med
Orano Med is a subsidiary of the Orano Group. Orano Med is a clinical-stage biotechnology company that develops a new generation of targeted therapies against cancer using the unique properties of lead-212 (212Pb), an alpha-emitting radioisotope and one of the more potent therapeutic payloads against cancer cells known as Targeted Alpha Therapy (TAT). Leveraging its unique and secured access to 212Pb, the company is developing several 212Pb-based radioligand therapies combined with various targeting agents. Orano Med has 212Pb manufacturing facilities, laboratories, and R&D centers in France and in the US and is currently expanding its GMP-manufacturing capacities for 212Pb radiolabeled pharmaceuticals in North America and Europe.
About RadioMedix
RadioMedix, Inc., a clinical-stage biotechnology company and former sponsor of the AlphaMedix trial, is based in Houston and Humble, Texas. The company is focused on innovative targeted radiopharmaceuticals for diagnosis, monitoring, and therapy of cancer. RadioMedix is developing radiopharmaceuticals for PET imaging and therapy (alpha- and beta-labeled agents). The company established contract service facilities for academic and industrial partners. including a cGMP and analytical suite for Phase I-II-III clinical trials and commercial launch. To learn more, visit www.radiomedix.com and LinkedIn.
About Sanofi
Sanofi is an R&D driven, AI-powered biopharma company committed to improving people’s lives and delivering compelling growth. We apply our deep understanding of the immune system to invent medicines and vaccines that treat and protect millions of people around the world, with an innovative pipeline that could benefit millions more. Our team is guided by one purpose: we chase the miracles of science to improve people’s lives; this inspires us to drive progress and deliver positive impact for our people and the communities we serve, by addressing the most urgent healthcare, environmental, and societal challenges of our time.Sanofi is listed on EURONEXT: SAN and NASDAQ: SNY
Head Office
125 avenue de Paris
92320 Châtillon
Tel. : +33 (0)1 34 96 00 00
Fax : +33 (0)1 34 96 00 01
Press Contact
Regina Jehle
Head of Communications and Public Affairs
regina.jehle@orano.group
Tel. : +33 6 74 56 11 31