 menu
menu
Preclinical Investigation of [212Pb]Pb-DOTAM-GRPR1 for Peptide Receptor Radionuclide Therapy in a Prostate Tumor Model
Abstract
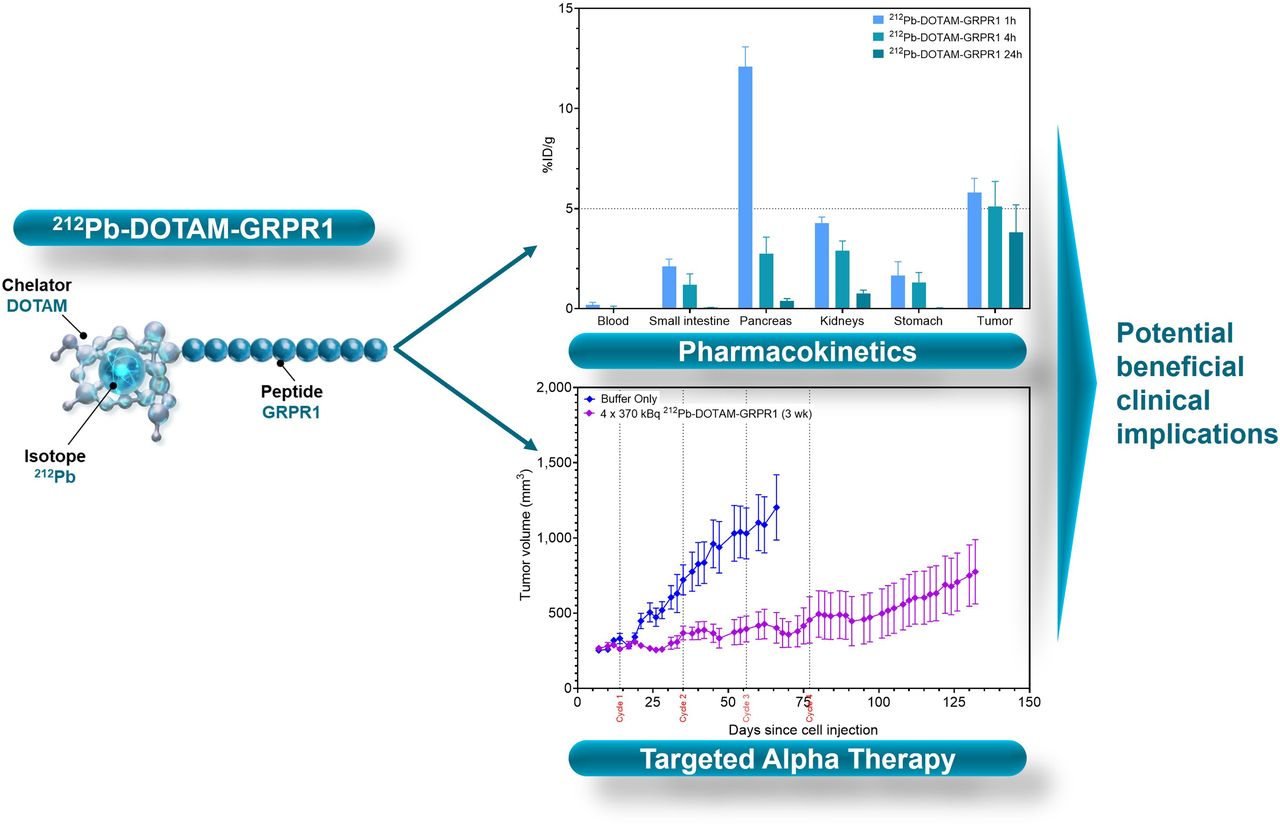
The role of gastrin-releasing peptide receptor (GRPR) in various diseases, including cancer, has been extensively studied and has emerged as a promising therapeutic target. In this study, we successfully achieved the use of [212Pb]Pb-DOTAM-GRPR1, comprising the α-particle generator, 212Pb, combined with a GRPR-targeting peptide, GRPR1, in a prostate cancer model.
Methods
Pharmacokinetics, toxicity, radiation dosimetry, and efficacy were assessed in GRPR-positive prostate tumor–bearing mice after intravenous administration of [212Pb]Pb-DOTAM-GRPR1 (where DOTAM is 1,4,7,10-tetrakis(carbamoylmethyl)-1,4,7,10-tetraazacyclododecane).
Results
Preclinical studies have shown tumor targeting of up to5 percent injected dose per gram over 24 h, and optimization of the drug formulation and quantity has led to minimized oxidation and off-target binding, respectively. Particularly, an increase in peptide amount from 28 to 280 ng was shown to reduce off-target uptake, especially at the level of the pancreas, by about 30%. Furthermore, dosimetry studies confirmed the kidney as the dose-limiting organ, and toxicity studies revealed that a nontoxic dose of up to 1,665 kBq could be injected into mice. Efficacy studies indicated a median survival time of 9 wk in the control group, which received only a buffer solution, compared with 19 wk in the group that received 4 injections of 370 kBq at 3-wk intervals.
Conclusion
Taken together, these combined data demonstrate the safety, tolerability, and efficacy of [212Pb]Pb-DOTAM-GRPR1, thus warranting further exploration in clinical trials.