menu
menu
Preclinical Evaluation of a Novel Clearing Agent-Independent Approach for Pretargeted Alpha Therapy with 212Pb
Aim
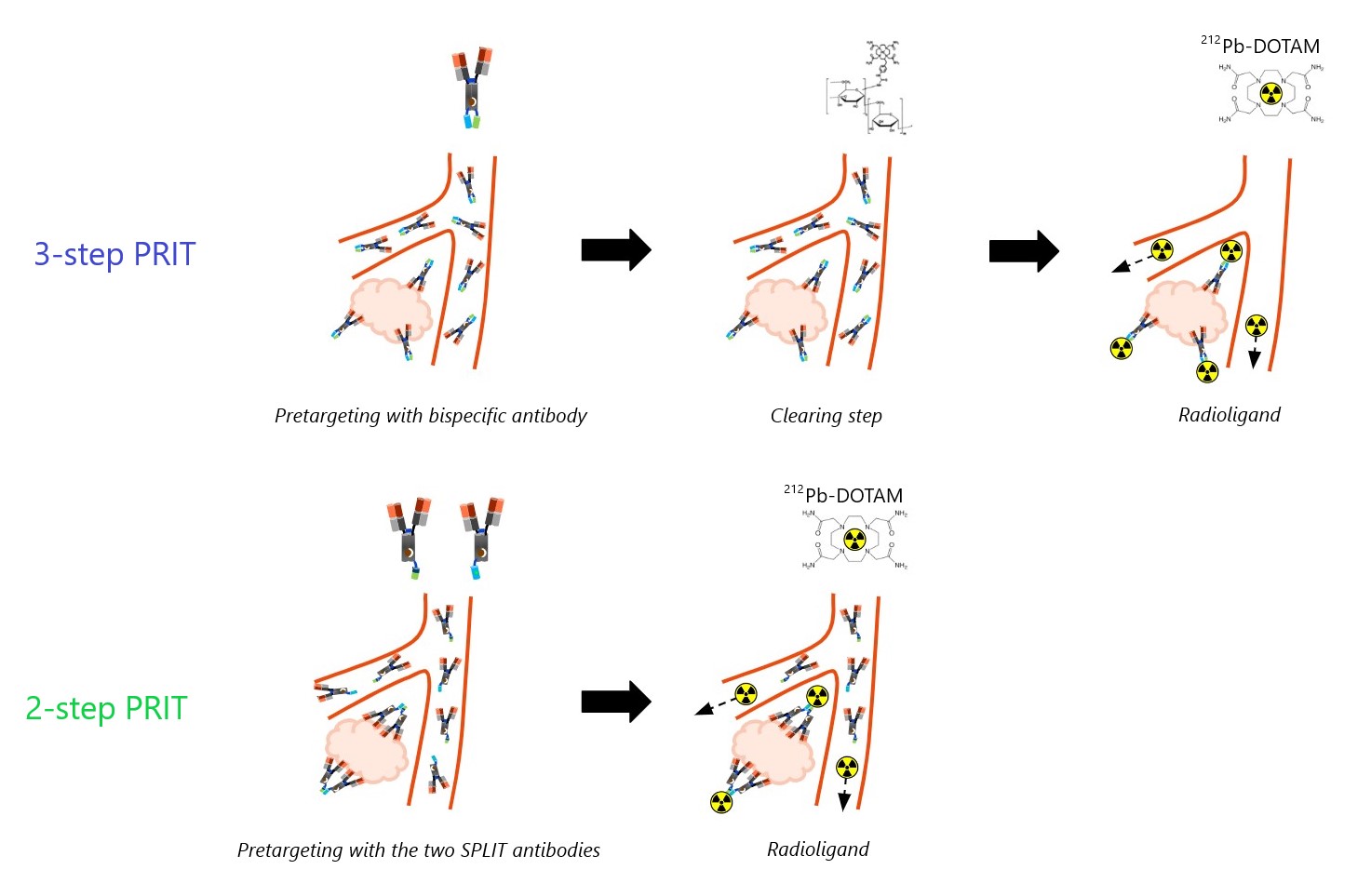
Over the years, various pretargeted radioimmunotherapy (PRIT) modalities have been proposed to enhance the therapeutic window of systemic radiotherapy. These approaches typically involve a tumour-targeting bispecific antibody (BsAb) that distributes within target tissue, followed by a small radiolabelled molecule that efficiently binds pretargeted sites or is quickly eliminated from the body. A common challenge has been ensuring rapid excretion of the radioactive payload while maintaining a high affinity for the slower-clearing pretargeting molecule. An intermediate step to clear or neutralise circulating pretargeting antibodies has often been employed to prevent off-tumour capture of the radioligand, but this adds complexity and safety risks, posing challenges to the clinical implementation of PRIT. Consequently, the field has shifted towards clearing agent-independent strategies, such as complementary oligomeric pretargeting and engineered bispecific antibodies.
Matching the biological half-life of the radioligand with the physical half-life of the radionuclide is particularly impactful using short-lived radionuclides such as 212Pb (t1/2 = 10.6 h), to minimise healthy tissue exposure while increasing the tumour absorbed dose. In this context, we developed a novel two-step, clearing agent-independent PRIT regimen for carcinoembryonic antigen (CEA)-positive tumours, involving a complementary bispecific antibody pair and a 212Pb radioligand. The efficacy and tolerability of this two-step PRIT approach were compared with a corresponding three-step PRIT scheme.
Materials and Methods
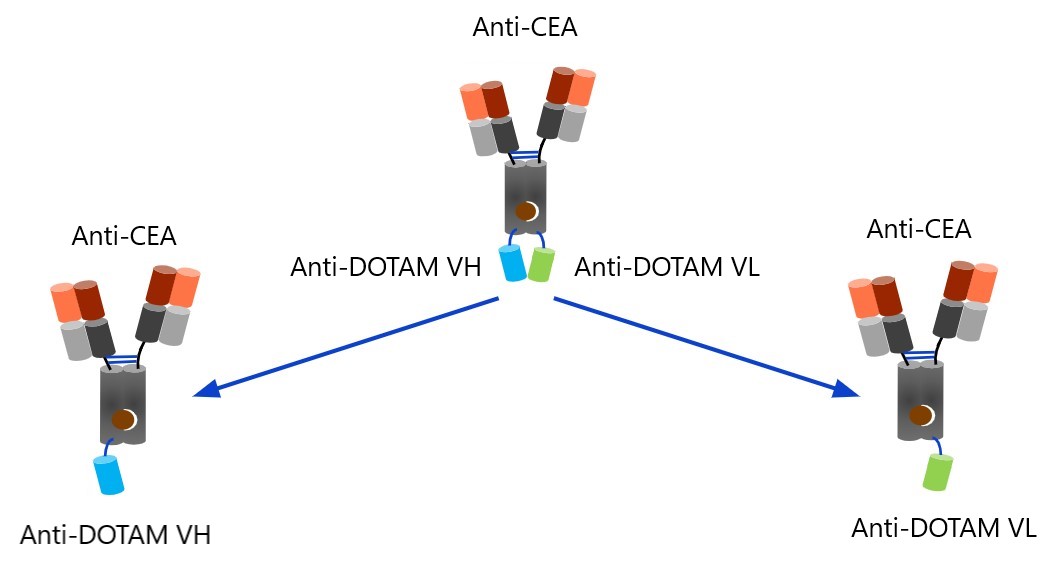
A technique was developed which relies on complementary "SPLIT" (SeParated v-domains LInkage Technology) pretargeting antibodies with specificity for CEA and a split high-affinity sub-pM 1,4,7,10 Tetrakis(carbamoylmethyl)1,4,7,10-tetraazacyclododecane (DOTAM) binder: one antibody carrying the anti-DOTAM VH and the other the anti-DOTAM VL domain. Independently, these antibodies are unable to bind
212Pb-DOTAM, as an active anti-DOTAM binding site is only generated in the presence of the complementary variable domain. This assembly is less likely to occur without target binding, but when tumour-bound, the split VH and VL domains form the complete binder in a concentration-dependent manner. Therefore, no clearing agent is required to prevent capturing of 212Pb-DOTAM in circulation.
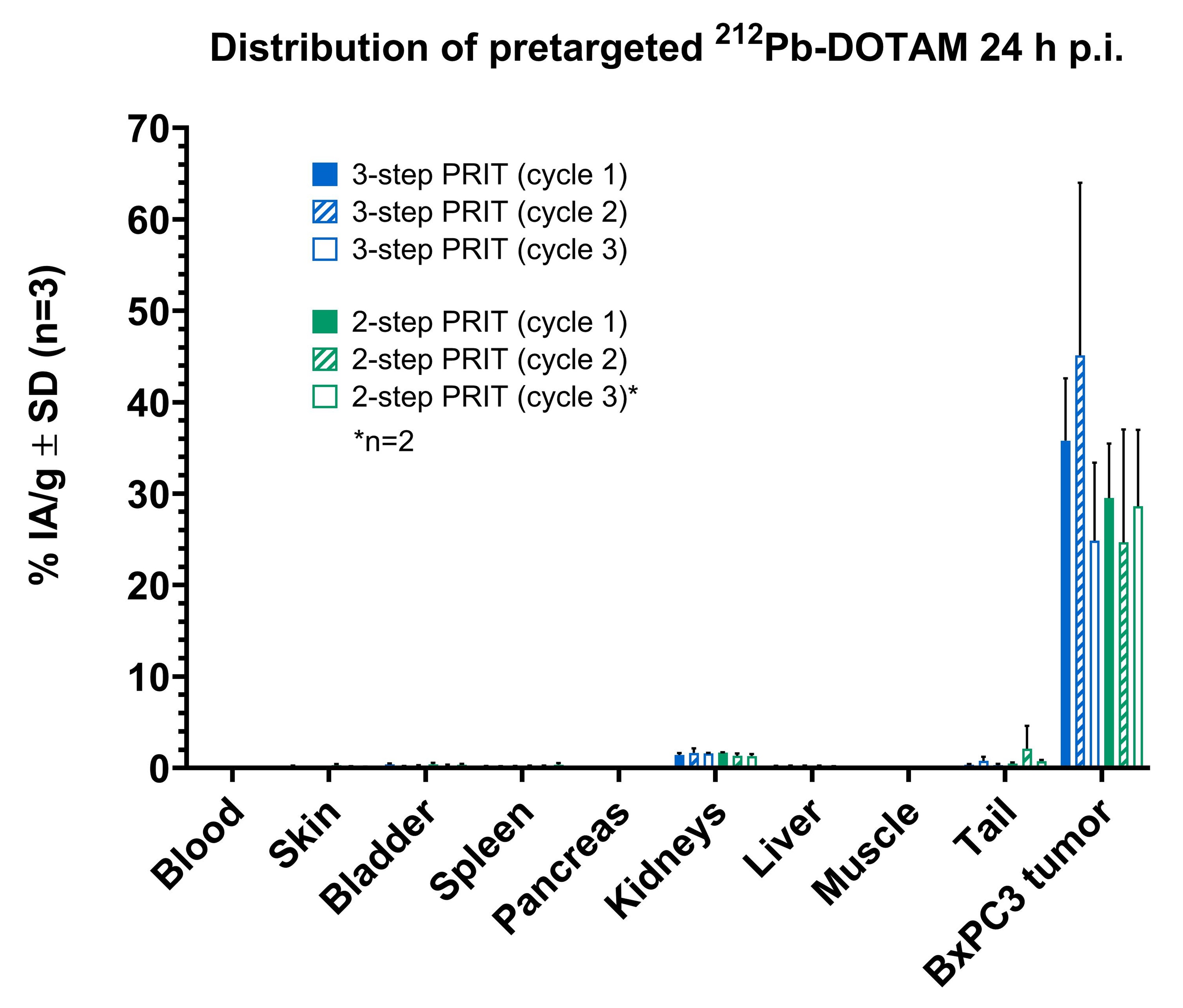
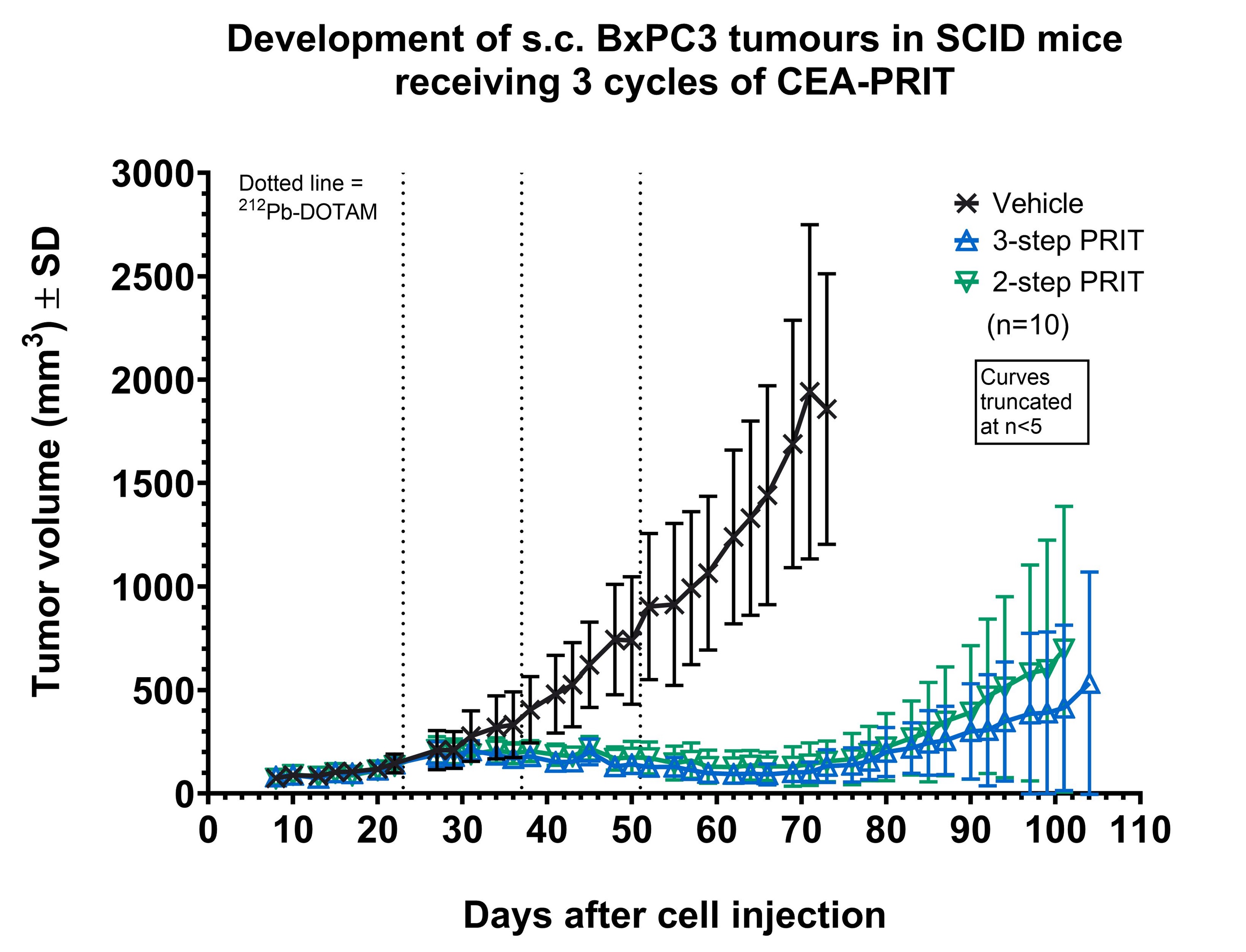
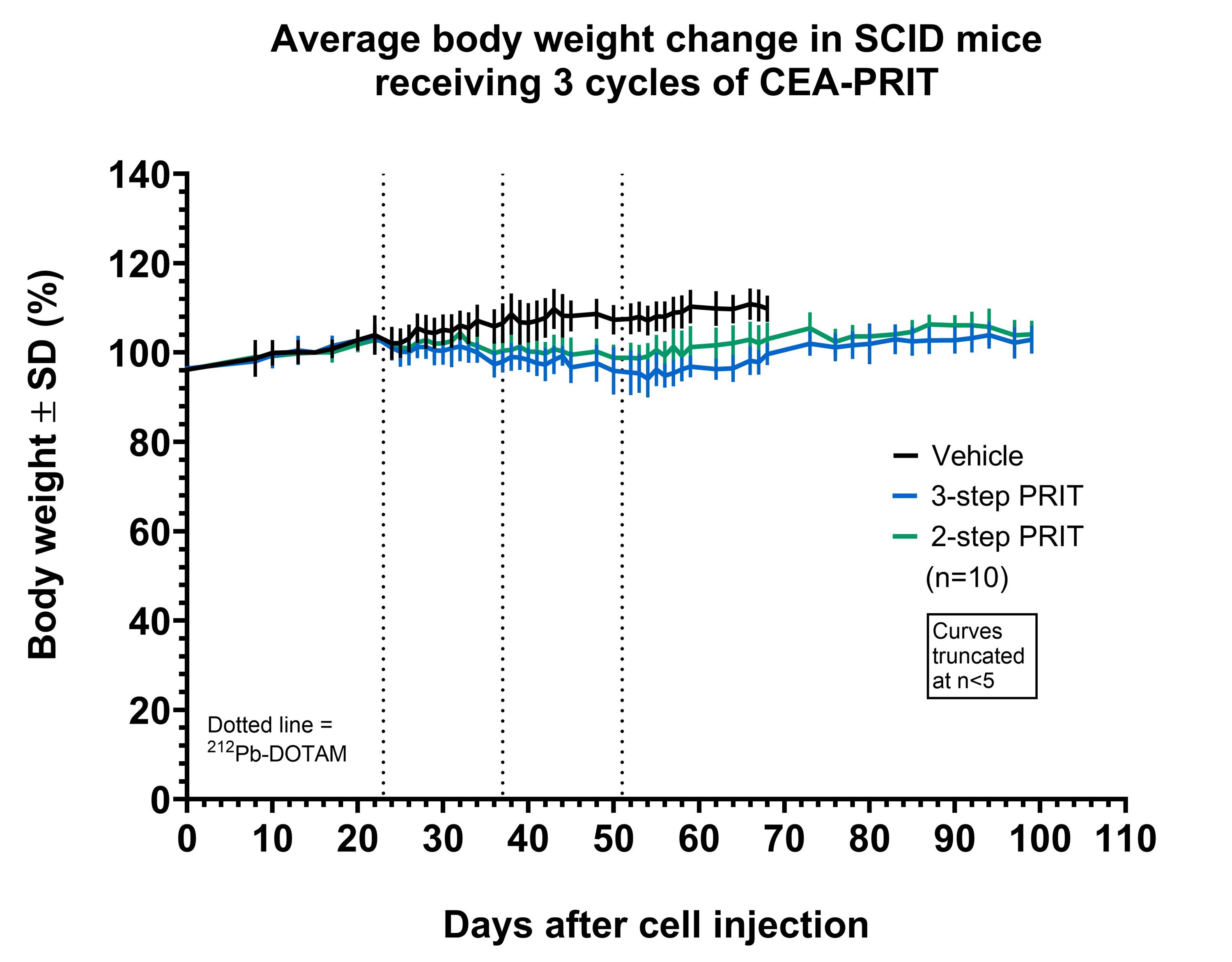
Severe combined immunodeficiency (SCID) mice bearing subcutaneous (s.c.) CEA-expressing BxPC3 xenografts were administered the two pretargeting antibodies via intraperitoneal injection, followed by 212Pb-DOTAM (0.74 MBq) administered intravenously 7 days later. Another group of SCID mice received a pretargeting CEA-DOTAM BsAb with a complete 212Pb-DOTAM binder. After 7 days, circulating BsAb was neutralised using a dextran-based clearing agent, followed by 212Pb-DOTAM (0.74 MBq) after 24 hours. Two-and three-step CEA-PRIT were compared in terms of 212Pb biodistribution, tumour growth inhibition, and tolerability after three treatment cycles as described above.
Results
Two- and three-step CEA-PRIT significantly and comparably delayed tumour growth, with mild transient body weight loss. Excellent 212Pb tumour specificity was confirmed, with an average tumour uptake of 25–30% of the injected activity per gram of tissue (IA/g) at 24 h p.i. for two-step PRIT and 36–45% IA/g for three-step PRIT, while the blood and kidney retention was <0.5% IA/g and <2 %IA/g, respectively, for both regimens. Thus, the novel two-step SPLIT PRIT approach successfully enabled the desired rapid excretion of the non-tumour bound 212Pb-DOTAM, while maintaining high and specific accumulation of 212Pb in tumour.
Conclusion
In vivo proof of mechanism and therapeutic efficacy was successfully demonstrated for two-step PRIT using the novel SPLIT pretargeting antibodies, rendering the logistically complex yet efficacious three-step PRIT regimen obsolete. Translation of this optimised SPLIT PRIT approach to a phase I trial is currently underway, offering potential improvements in clinical PRIT implementation.