menu
menu
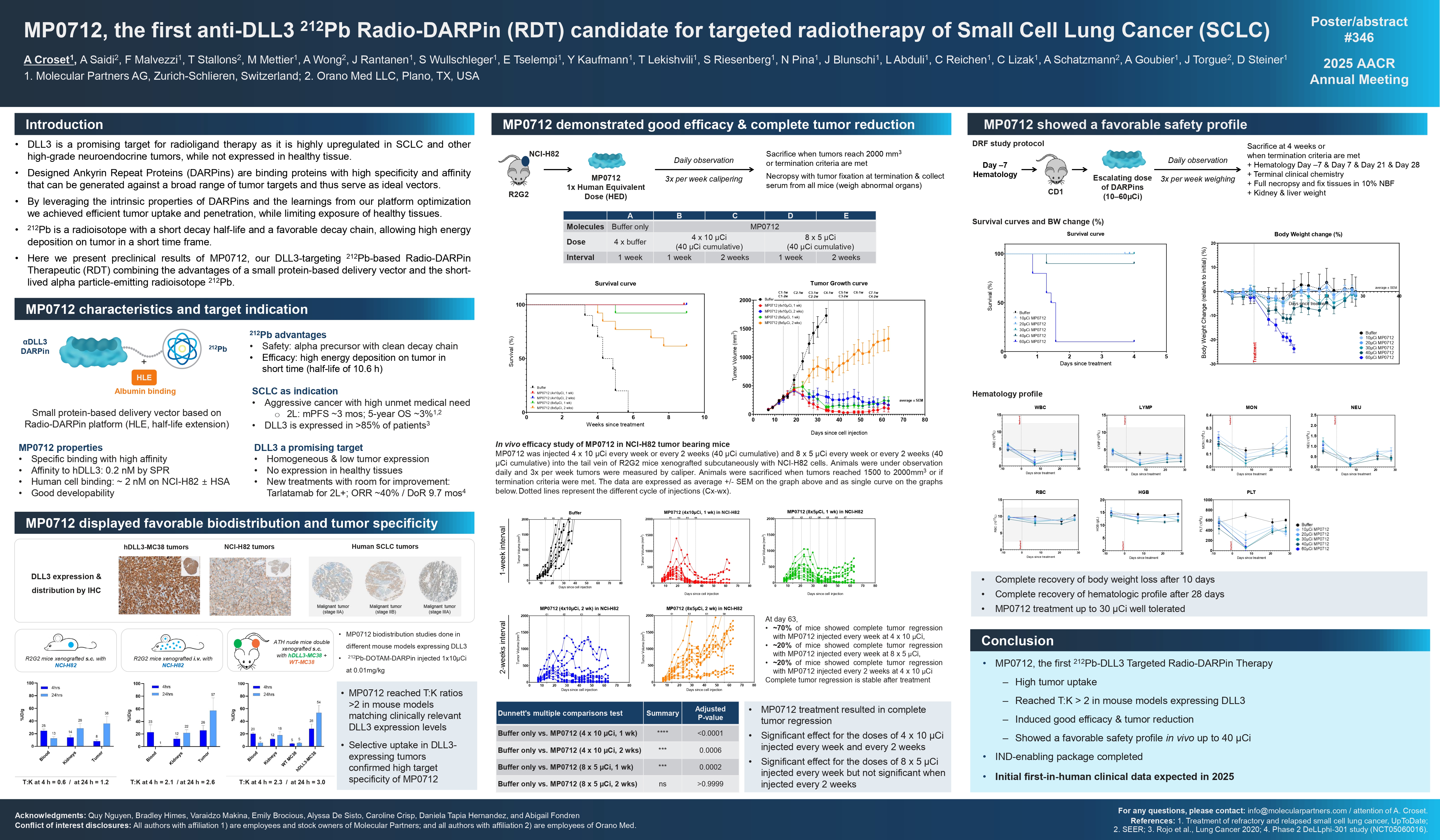
MP0712, the first anti-DLL3 ²¹²Pb Radio-DARPin (RDT) candidate for targeted radiotherapy of Small Cell Lung Cancer (SCLC)
Abstract:
Radioligand therapy has shown strong clinical potential for the treatment of certain neuroendocrine and prostate tumors. Nevertheless, effective strategies in various cancer types are currently restricted by the lack of appropriate targeting agents for suitable tumor-associated antigens. Not expressed in healthy tissue, DLL3 is one of these promising targets highly upregulated in SCLC and other high-grade neuroendocrine tumors. Herein we present preclinical results of our DLL3-targeting ²¹²Pb-based Radio-DARPin Therapeutic (RDT) combining the advantages of a small protein-based delivery vector and the short-lived alpha particle-emitting radioisotope ²¹²Pb. Designed Ankyrin Repeat Proteins (DARPins) are a class of binding proteins with high specificity and affinity that can be generated against a broad range of tumor targets. Leveraging the learnings from our DARPin platform optimization allowed us to achieve efficient tumor uptake and penetration, while limiting exposure of healthy tissues. Our Radio-DARPin platform was optimized to effectively reduce DARPin uptake to kidneys, a major drawback of all small-size polypeptide vectors. This can be addressed by engineering the DARPin scaffold surface in conjunction with half-life extension (HLE) through serum albumin binding. ²¹²Pb is a radioisotope with a short half-life of 11h and a favorable decay chain, allowing high energy deposition on tumor in a short time frame. By combining ²¹²Pb with a HLE anti-DLL3 DARPin candidate, we aimed to generate a targeted RDT with high efficacy and a favorable safety profile. After several screening rounds of anti-DLL3-binding DARPins combined with HLE in mice, MP0712 was selected as lead candidate for further development. This molecule showed specific binding to lung cancer cells in vitro (~2nM on NCI-H82 ± HSA) and high affinity to human DLL3 (0.2nM by Surface Plasmon Resonance). MP0712 biodistributions were assessed in different mouse xenograft tumor models, matching clinically relevant DLL3 expression levels or overexpression of DLL3, and reached tumor:kidney ratios >2. Double xenografted mouse models showed selective uptake in DLL3-expressing tumors compared with tumors not expressing DLL3, confirming high target specificity of MP0712. The anti-tumor activity of MP0712 in different mouse xenograft tumor models showed promising efficacy. MP0712 led to tumor stabilization and significant effects on tumor versus necrotic tissue. In NCI-H82 tumors, median survival duration increased up to 3-fold with MP0317 compared with the buffer control. MP0712 also showed a favorable safety profile in vivo up to 30μCi. These preclinical results support our ²¹²Pb-based RDT against DLL3 as a promising treatment option for SCLC, with encouraging in vivo antitumor activity and a good safety profile for our first DLL3-targeting ²¹²Pb-RDT candidate for further development into the clinic.
Conclusion:
- MP0712, the first 212Pb-DLL3 Targeted Radio-DARPin Therapy
- High tumor uptake
- Reached T:K > 2 in mouse models expressing DLL3
- Induced good efficacy & tumor reduction
- Showed a favorable safety profile in vivo up to 40 μCi
- IND-enabling package completed
- Initial first-in-human clinical data expected in 2025